Volatile Oil from Western Juniper
E.F. Kurth
J.D. Ross
Report No. C-3
April 1954
Oregon Forest Products Laboratory
State Board of Forestry and School of Forestry,
Oregon State College, Cooperating
Corvallis
Table of Contents
Summary
Thirty-two charges of wood from western juniper trees were steam distilled. The volatile oil was recovered, and its composition and yield determined.
The wood was reduced to various-size particles by chipping and by hammer-milling. Some charges contained bark; some were of wood only.
Low steam pressures (under 10 psi) and hammer-milled chips gave oil yields of higher quality and greater volume.
Oil recovery averaged around 1.4 percent, of which about 15-40 percent was cedrol.
Return to Table of Contents
Introduction
In large areas of central and eastern Oregon the principal tree species is western juniper (Juniperus occidentalis Hook.). The principal uses for the wood are confined at present to fence posts, novelties, and fuel. Most large trees are decayed internally by fungus attack, making difficult the recovery of lumber or pencil stock.
In an effort to find high-value products from the species which would permit its economic utilization, an investigation of its properties and constituents was initiated at the Oregon Forest Products Laboratory.
The present report is a study of steam distillation of the volatile oils in the wood.
Previous studies of western juniper wood included an investigation in 1947 of the amount and composition of the extractives in the wood (Kurth and Lackey, 1948). This investigation showed yields of 0.9-1.25 per cent of volatile oil (based on the weight of dry wood), with the main constituents being cedrol and cedrene. These materials are also present in commercial cedarwood oil recovered from eastern redcedar heartwood (J. virginiana) and species of juniper (J. ashei Buchholz, J. flaccida Schlecht., and J. monosperma Sarg.) growing in Texas and Northern Mexico. Other extractives found in western juniper were fatty and resin acids, phytosterol, resenes, and a catechol phlobaphene.
A further study of the volatile-oil content of juniper trees was made in the fall of 1951 on trees growing on moist ground along stream beds, dry ground, and arid lava-ash-type soil east and south of Prineville, Oregon (Burnet, 1954). In this investigation, five trees about 16 inches in diameter two feet above the ground were felled and sampled for each type soil; cross-sectional disks were then cut from the butt logs and the top logs to obtain information on the distribution of the oil within the tree trunk and under different growing conditions. The yield of volatile oil from these trees was determined in the Laboratory and is given in Table 1.
Table 1. Yield of Volatile Oil: from Juniper Trees
Growing on Different Types of Soil.
| Soil type | Volatile Oil | |
| Butt1 | Top2 | |
| Percent3 | Percent3 | |
| Moist | 2.43 | 1.12 |
| Dry | 2.35 | 1.01 |
| Arid lava-ash | 2.01 | 1.14 |
1 Two feet above ground level
2 Six-inch top diameter
3 On an ovendry weight basis
The data show that the yield of volatile oil from all of the trees was similar; the yield of oil from the wood at the 2-foot above-ground height varied from 2.01 to 2.43 percent, whereas that from the top of the trees varied from 1.01 to 1.14 percent. The average yield at the bottom of the trees was 2.26 percent and that from the top of the trees was 1.09 percent, giving an average yield for top and bottom disks of 1.73 percent.
In June 1952, the John D. Walsh Company, New York City, advised the Laboratory that the price of cedrol was from 3.50 to 4.00 dollars a pound and requested that crude and refined samples of the oil steam-distilled from western juniper wood be submitted to them for market evaluation. In this connection, fresh lots of juniper wood were obtained, the wood was then chipped or hogged, and the chipped or hogged material was steam-distilled at various steam pressures and for different periods of time to ascertain the optimum conditions of oil recovery. Samples of the oil were submitted for market evaluation and the use of the oil-free steamed wood residue was explored for fiberboard production. The purpose was to establish whether an integrated operation combining oil recovery with use of the spent wood residue for fiberboard production would be economically feasible.
Return to Table of Contents
Experimental Procedure
The first lot of juniper wood was collected by Laboratory personnel in the Prineville, Oregon area during the latter part of May 1953. Living trees were felled and cut into 4-foot lengths. These were hand-barked, slabbed, and chipped into conventional pulp-size chips. Where necessary they were further sized by passing through a chip breaker. A part-of the chips was further processed by hammer-milling to ascertain the effect of particle size on the rate of oil distillation.
The influence of particle size on oil yield was explored on a small scale in a Clevenger apparatus. The results on 1/2-inch wood chips, hammer-milled chips, and Wiley-milled chips passing a 1/2-inch screen are shown in Table 2. It was observed that large chips gave substantially lower yields of oil than did fine material.
Table 2. Effect of Particle Size on Oil Yield in a Clevenger Apparatus
| Material | Time of distillation | Volumetric yield | Gravimetric yield |
| Hours | Percent1 | Percent1 | |
| 1/2" chips | 20 | 0.63 | 0.40 |
| Hammer-milled chips | 30 | 1.08 | 0.85 |
| Wiley-milled chips | 30 | 1.20 | 0.92 |
1 Dry wood basis
The Laboratory's pulp digester, with some modifications, was used for the large-scale steam distillations of juniper wood. Steam was admitted at the bottom of the digester, passed up through the bed of wood chips, and the vapors ware vented from the top of the digester through a 50-foot spiral of 3/8-inch copper tubing contained in a 10-gallon metal can through which cooling water was passed.
Return to Table of Contents
First Series of Distillations
The condensate was collected in a 6-liter aspirator bottle filled with 1/4-inch Raschig rings, water, and hexane as shown in Figure 1. The oil dissolved in the hexane, which was drained off at the end of a run and fractionally distilled to leave the crude oil. Some of the oil was lost along with some hexane through incomplete separation of the hexane from the water layer.
In operation, the digester was preheated for 15-30 minutes with the cover off, then loaded with wood from which a composite sample had been taken for moisture determination. The digester was then closed, inverted, and the condenser connection attached. Condenser cooling water was started and steam admitted to the charge according to a set pressure regulated by a temperature controller attached to the digester. Rate of vapor discharge was regulated by manual control of a valve in the vent line. Condensed vapors were conducted through the hexane trap, and waste water was collected and weighed as the distillation progressed. At the end of the distillation, usually 4-6 hours, the condenser was shut off and the condensate temperature allowed to rise in order to flush out any oil trapped in the condenser. After this, the hexane was drained off and distilled in a laboratory apparatus to leave the crude oil. After standing overnight, the waste water was skimmed with paper to recover traces of oil; this was then added to the main sample of oil.
Data for all runs are compiled in Table 3. The yields of oil increased with increase in steam pressures. Some of the yields were rather irregular; this may be attributed to (1) channeling of steam through the charge, (2) differences in proportions of heartwood and sapwood in the charges, (3) possible retention of oils in the condenser from one run to another and (4) incomplete separation of the oil from the aqueous condensate.
Rate of vapor venting was not investigated to any extent because of its irregularity and the inability to control it satisfactorily. It appears customary to use considerably more than theoretical amounts of steam in volatile oil distillations, so this practice was followed (3, Volume I, 159-166).
In runs 10, 11, 12, the hexane trap was drained at the end of the first hour, the third hour, and at the end of the run. The crude oil was recovered and an approximate distillation rate established. The results of these runs are shown in Table 4. These values do not appear reliable, probably because of appreciable quantities of oil held up in the condenser. The values for rate of distillation shown in Figure 4 are considered more representative.
Table 3. Data from Steam Distillation of Western Juniper Wood; First Series
| Run | Material | Wet wt. of charge | Moisture content | Dry wt. of charge | Steam pressure | Distillation time | Wt. of condensate | Wt. of crude oil | Oil yield |
| Lb | Percent1 | Lb | psi | Hr/ Min | Lb | Lb | Percent2 | ||
| 1 | Chips3 | 20 | 53.3 | 9.3 | 0 | 3:05 | Discarded | -- | |
| 2 | Chips | 57 | 55.5 | 25.3 | 5 | 6:00 | 214 | 0.06 | 0.24 |
| 3 | H.M. Chips4 | 60 | 42.1 | 34.6 | 35 | 4:45 | 392 | 0.386 | 1.11 |
| 4 | H.M. Chips | 60 | 49.5 | 30.3 | 15 | 6:15 | 358 | 0.114 | 0.38 |
| 5 | H.M. Chips | 54 | 43.2 | 30.6 | 60 | 4:10 | 453 | 0.446 | 1.46 |
| 6 | H.M. Chips | 56 | 42.8 | 32.0 | 5 | 6:00 | 402 | 0.271 | 0.85 |
| 7 | Chips | 56 | 43.2 | 31.7 | 25 | 6:00 | 492 | 0.334 | 1.05 |
| 8 | H.M. Chips | 50 | 49.2 | 25.4 | 5 | 6:00 | 254 | 0.111 | 0.44 |
| 9 | H.M. Chips | 50 | 34.0 | 33.0 | 40 | 6:00 | 356 | 0.60 | 1.82 |
| 10 | H.M. Chips | 45 | 36.9 | 28.4 | 40 | 5:45 | 427 | 0.35 | 1.23 |
| 11 | H.M. Chips | 41 | 37.5 | 25.5 | 20 | 5:45 | 422 | 0.37 | 1.45 |
| 12 | H.M. Chips | 39 | 31.5 | 26.6 | 0 | 6:20 | 241 | 0.201 | 0.76 |
| 13 | Chips | 64 | 34.5 | 42.0 | 25 | 5:15 | 376 | 0.396 | 0.945 |
| 14 | Chips | 60 | 33.7 | 39.7 | 45 | 5:35 | 368 | 0.302 | 0.76 |
| 15 | Chips | 42 | 32.8 | 28.2 | 40 | 6:10 | 439 | 0.312 | 1.11 |
| 16 | Chips | 52 | 16.5 | 43.4 | 60 | 8:00 | 519 | 0.464 | 1.07 |
| 17 | H.M. Chips | 34 | 18.3 | 27.8 | 60 | 8:00 | 596 | 0.209 | 0.75 |
1 On the wet basis
2 On the ovendry basis
3 Chips - Conventional 1/2-inch pulp chips
4 H.M Chips - hammer-milled chips
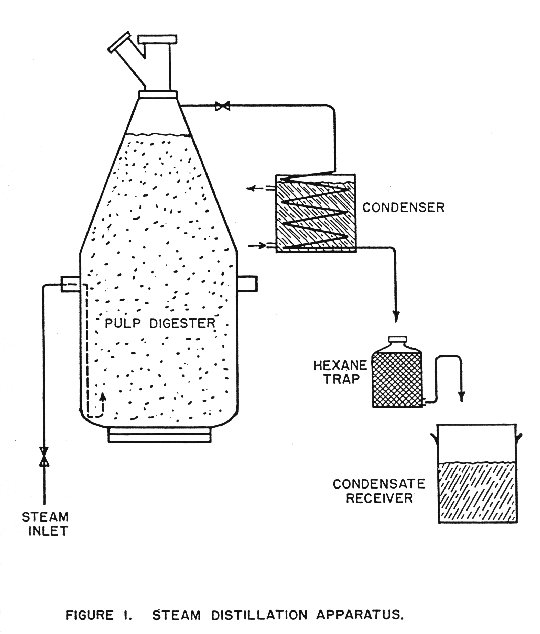
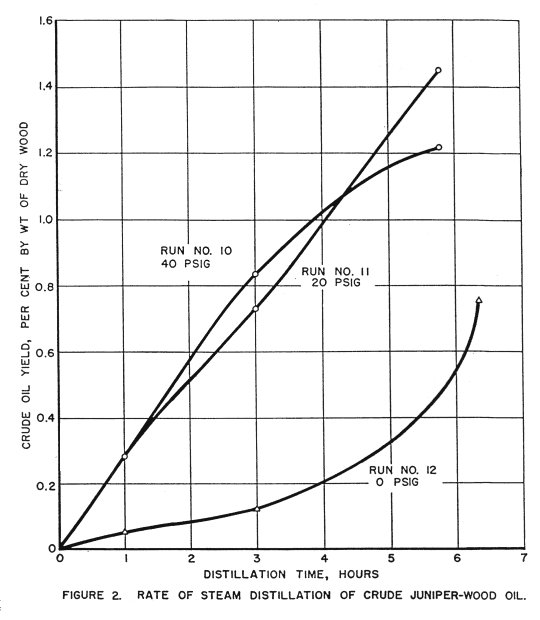
Table 4. Rate of Steam Distillation
| Run | Dry wood | Steam pressure | Oil Recovered | Overall yield | ||
| Lb | Psi | Lb | Lb | Lb | Percent1 | |
| 10 | 28.4 | 30 | 0.082 | 0.155 | 0.113 | 1.23 |
| 11 | 25.5 | 20 | 0.073 | 0.114 | 0.18 | 1.45 |
| 12 | 26.6 | 0-1 | 0.0154 | 0.0187 | 0.168 | 0.76 |
1Ovendry weight basis
Rate curves for these runs are shown in Figure 2.
Runs 2, 7, 13, 14, 15 were made on whole chips, primarily to supply material for fiberboard tests. The yields appeared to be slightly lower than from hammer-milled chips, although the difference was not so great as was expected. No determination of distillation rate was made on whole chips, since this distillation appeared to be slower than that of the hammer-milled chips.
Return to Table of Contents
Examination of the crude oil
The variation in oil yields indicated that there might be some difference in oil composition obtained by various steam temperatures. Samples of crude oil were therefore vacuum distilled to determine any substantial difference in composition.
In each case, 50 grams of crude oil were vacuum distilled at 10 mm pressure. Material balances for these distillations are shown by Table 5.
Table 5. Material Balances for Vacuum Distillations.
| Oil from run | Distillate | Residue | Loss | Recovery |
| Grams | Grams | Grams | Percent1 | |
| 5 (60 psi) | 40.59 | 4.17 | 5.24 | 81.5 |
| 6 (5 psi) | 42.13 | 5.73 | ??? | ??? |
| 8 (5 psi) | 40.93 | 3.29 | 5.70 | 82.0 |
| 9 (40 psi) | 37.27 | 3.03 | ??? | ??? |
By observation of the temperature and the nature of the distillate, it was possible to estimate the yields of cedrene and cedrol. In each case, the cedrol fraction appeared to come over at 100 deg C and above. The low-pressure runs yielded an oil having higher cedrol content than that from high-pressure runs. Since the total oil yield was higher for high-pressure runs, the overall cedrol yield was higher for these pressures.
This situation is indicated by a rough calculation of the cedrol-fraction yields shown in Table 6. For this approximation, specific gravities of 0.94 were used for all fractions, and 100 deg C was taken as the division between cedrol and cedrene.
Table 6. Approximate Calculation of Cedrol Content of
Various Oils from Vacuum-distillation Data.
| Run | Steam pressure | Low-boiling fraction | High-boiling fraction | Cedrol content | Cedrol yield | ||
| Volume | Weight | Volume | Weight | ||||
| Psi | ml | Grams | ml | Grams | Percent1 | Percent2 | |
| 5 | 60 | 26.0 | 24.4 | 14.0 | 13.21 | 35.0 | 0.51 |
| 6 | 5 | 23.5 | 22.1 | 20.5 | 19.3 | 46.5 | 0.395 |
| 8 | 5 | 13.0 | 12.2 | 28.0 | 26.3 | 68.2 | 0.30 |
| 9 | 40 | 23.5 | 22.1 | 16.0 | 15.0 | 40.5 | 0.736 |
1Based on weight of crude oil.
2Based on dry weight of wood.
While this calculation is admittedly approximate, it does indicate that the low-pressure oils were richer in cedrol, but high pressures gave better overall yield of cedrol.
Three samples of juniper oil were submitted to the John D. Walsh Company for evaluation. The samples and their properties are shown in Table 7. This company reported that all three samples showed high cedrol contents, but that they had an odor significantly different from that obtained from the mexicana (ashei) or virginiana species of juniper. Specifically, the oil was reported to have a greasy, unpleasant odor which repeated distillation did not remove.
Table 7. Properties of Volatile Oil Samples Sent
to the John D. Walsh Co.
| Sample | Refractive index at 20 deg C | Ester (cedryl acetate) content | Alcohol content |
| Percent | Percent | ||
| 1. Crude oil | 1.4988 | 10.3 | 48.1 |
| 2. Vacuum-distilled oil | 1.5075 | 5.4 | 45.2 |
| 3. Cedrol fraction | 1.5125 | 10.4 | 84.4 |
Second Series of distillations
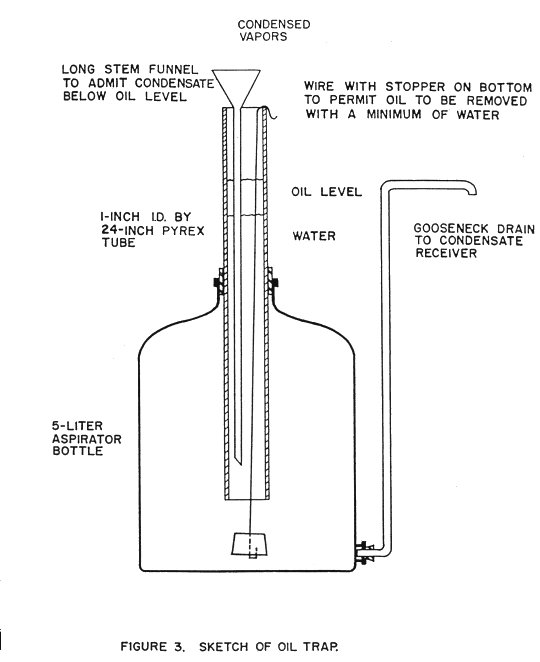
Additional work was undertaken with the purpose of eliminating the greasy odor from the oil, ascertaining the suitability of mechanical fiber prepared the steamed wood residue, and obtaining further information on the best operating conditions for oil recovery. Another lot of juniper wood was collected in October 1953 which contained some heartwood rot. The hexane trap previously used to recover the oil was discarded, and a water trap installed. This trap, shown in Figure 3, permitted continuous observation and measurement of the oil as it collected and thus the rate of distillation could be calculated.
Operating procedure was the same as before. The digester was preheated, loaded with a weighed charge, and steam passed through at controlled temperature and rate for the desired length of time. Samples of the charge were oven-dried to determine dry weight of the charge. The oil, recovered with some water, was placed in a separatory funnel until a clear separation was obtained, then removed and weighed.
The variables involved were (1) particle size of wood, (2) digester steam pressure, (3) rate of steam flow, (4) distillation time, and (5) presence or absence of bark in the charge.
Only two particle sizes were used in the new series of runs, since the spent material was to be used for board products. These were (1) standard 1/2-inch pulp chips suitable for fiber production and (2) hammer-milled chips for particle-board production.
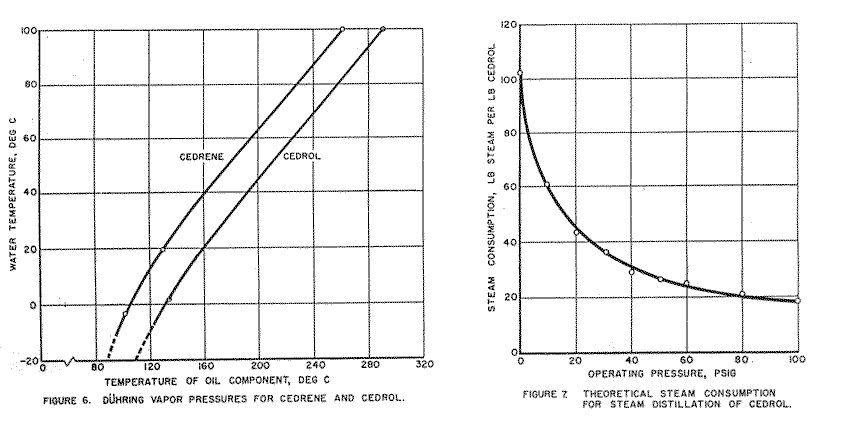
The operating steam pressure was varied from 10 to 50 psi. Calculations, included in the appendix, on theoretical steam requirements for distillation of cedrol, indicated that 50-60 psi was probably the practical maximum pressure.
The rate of steam flow was reduced considerably for this work over that previously used. The rate of steaming is relatively unimportant, except from the economic standpoint, since the theoretical consumption is necessarily exceeded several-fold in practice. While the theoretical steam consumption for distillation of cedrol is about 20-25 pounds of steam per pound of oil at 50-60 pounds per square inch, the actual consumption in practice for recovery of cedarwood oil is around 100 lb/lb oil (3, Vol 1, p.159). This figure was approached in some of the runs in this series.
The time of distillation was not investigated to any extent in this series of runs, for the new oil trap permitted visual observation of the rate of oil recovery. Therefore, it was possible to cut off a distillation when oil recovery became small.
The charges were steamed both with and without bark. It is questionable whether much distinction can be made in the two types of charges, for the juniper logs were rather difficult to peel and there was some bark (especially inner bark) left in the irregularities of the logs. It is probable that any commercial operation will use both wood and bark because of the difficulty in barking the twisted and irregular shapes that commonly occur in juniper logs.
Some 15 runs were made; a tabulation of pertinent data is shown in Table 8.
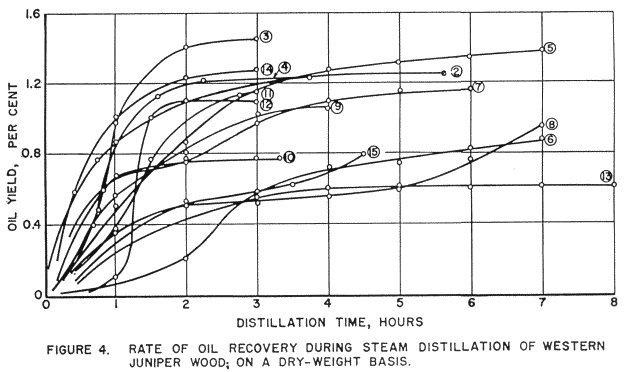
Figure 4 shows the relationship between the rate of oil recovery and the time of distillation.
The first 5 runs were made with hammer-milled wood, the remaining 10 runs on chips. It will be observed that the yields and distillation rates are higher for the smaller particle sizes. This difference is more pronounced at lower steam pressures and tends to diminish at higher pressures.
It appeared that the presence of bark in the charge reduced the oil yield slightly; however, the experimental results were too erratic to draw a definite conclusion.
As previously observed, higher yields and faster distillation were achieved with higher steam pressures. It appeared, however, that at any pressure, the bulk of the oil was recovered within the first 3-4 hours.
The steam consumption, as far as oil recovery was concerned, was relatively unimportant. The minimum rate of steaming in this case was dictated by the amount of flow necessary to keep the condenser open. It was possible to operate with a rate of about 100-150 pounds of steam per pound of oil, which conforms to commercial practice. Run 13 used a high excess of steam. There was no apparent difference in the characteristics of the run with the exception that the oil was more difficult to trap because of dispersion in the large amount of water. The steam consumption also became high and somewhat unrealistic when the distillation was continued beyond a practical length of time.
Return to Table of Contents
Examination of the crude oil
The crude oil, as recovered, was a dark-red, opaque liquid. The odor was somewhat harsh, suggestive of the odor of the foliage or bark. There was no apparent difference in odor between oil from bark-free and bark-included charges.
Table 8. Data from Steam Distillation of Western Juniper Wood; Second Series
| Run | Material1 | Wet wt. of wood | Moisture content | Dry wt. of wood | Steam pressure | Distillation time | Wt. oil recovered | Yield | Steam/ oil |
| Lb | Percent2 | Lb | psi | Hours | Lb | Percent | Lb/lb | ||
| 1 | hm-wob | 57.5 | 44.0 | 32.1 | 50 | 6.5 | 0.53 | 1.65 | 240 |
| 2 | hm-wb | 52.0 | 46.0 | 28.1 | 50 | 5.75 | 0.353 | 1.26 | 246 |
| 3 | hm-wob | 44.0 | 37.5 | 27.4 | 50 | 3 | 0.398 | 1.46 | 126 |
| 4 | hm-wb | 48.0 | 43.0 | 27.4 | 50 | 3.75 | 0.34 | 1.24 | 246 |
| 5 | hm-wb | 40.0 | 37.0 | 25.0 | 10 | 7 | 0.345 | 1.38 | 385 |
| 6 | chips-ns-wob | 39.0 | 45.0 | 21.4 | 10 | 7 | 0.187 | 0.88 | 700 |
| 7 | chips-ns-wob | 49.0 | 36.5 | 31.0 | 50 | 7 | 0.358 | 1.16 | 328 |
| 8 | chips-ns-wb | 42.0 | 43.0 | 23.9 | 30 | 7 | 0.230 | 0.96 | 505 |
| 9 | chips-s-wob | 42.0 | 41.5 | 25.8 | 30 | 4 | 0.274 | 1.07 | 224 |
| 10 | chips-s-wb | 39.0 | 41.5 | 22.8 | 50 | 3.33 | 0.176 | 0.78 | 305 |
| 11 | chips-s-wob | 44.0 | 38.5 | 27.0 | 50 | 3 | 0.313 | 1.16 | 144 |
| 12 | chips-s-wob | 46.0 | 40.5 | 27.4 | 50 | 3 | 0.30 | 1.10 | 146 |
| 13 | chips-s-wob | 41.0 | 39.5 | 24.8 | 10 | 8 | 0.15 | 0.61 | 2060 |
| 14 | chips-ns-wb | 49.0 | 37.5 | 30.5 | 50 | 4 | 0.39 | 1.28 | 118 |
| 15 | trash chips | 33.0 | 35.0 | 21.5 | 10 | 4.5 | 0.172 | 0.80 | 495 |
1 hm - hammer-milled
wob - without bark
wb - with bark
ns - not screened
- screened
2 On a wet-weight basis
A number of samples, when exposed to air for several days, developed a dark blue-green color, apparently because of copper contamination from the condenser. This color was removed by treatment with citric acid as described by Guenther (1949, p. 311 Vol. I).
Oils obtained from runs using 10 psi steam pressure and less deposited cedrol crystals on standing overnight. Samples of oil from runs 5 and 6 reacted in this manner. Filtration of the samples showed 17.4 percent of the weight of oil deposited as cedrol in run 5, and 10 percent from run 6. The other low pressure runs, 13 and 15, did not give crystalline cedrol. Run 13 used a high excess of steam which resulted in partial emulsification of the oil and water which seemed to inhibit crystallization. Run 15 used waste wood with considerable bark, and this did not deposit crystalline cedrol.
The oils from high-pressure runs, when treated by the Raybak process (Guenther, 1949, p. 300 Vol I) yielded about 20-25 per cent of the weight of oil as crystalline cedrol.
A number of random treatments of the oil were tried in an effort to improve the odor of the oil as requested by the John D. Walsh Co, 32 Broadway, New York, New York.
Samples treated in the following manner were sent to this firm for examination:
- 1. Crude oil as recovered, not treated.
- 2. Oil extracted with citric acid to remove heavy metals.
- 3. Oil extracted with (1) citric acid and (2) with hot, saturated, sodium bisulfite solution.
- 4. Oil refluxed with 5 percent sodium hydroxide solution.
- 5. Oil extracted with 10 percent hydrochloric acid.
According to the Walsh Company, the above treatments did not result in any noticeable improvement in the odor of the oil. It therefore appeared that the oil from western juniper had somewhat different properties from the other cedarwood oils. This suggested two further avenues of research if this project were continued. The oil could either be modified to duplicate the properties of established cedarwood oils, or product research could be carried out to find uses for the western juniper oil in its natural condition.
Return to Table of Contents
Remarks
The following remarks can be made regarding operating conditions for volatile oil recovery:
1. For maximum oil yields at the most favorable rate, distillation at 50-60 psi is recommended.
2. If oil recovery is the only purpose, hammer-milled wood will be better than chips because of the higher oil yield and faster recovery.
3. The use of steam pressures under 10 psi gave oils that deposited crystalline cedrol on standing. It was possible to recover 10-15 percent of the weight of the oil as solid cedrol by filtration. The cedrol was quite pure and could easily be recrystallized from hot ethanol if higher purity was desired. This product had a pleasant odor.
4. Oils from high-pressure runs when treated by the Raybak method yielded 20-25 per cent cedrol. It was more difficult to purify this cedrol, however, than that from low-pressure runs.
5. Minimum steam consumption for the range of pressures investigated appeared to be 100-150 pounds per pound of oil recovered.
The following generalizations outline probable economics of juniper oil production:
1. Average oil recoveries from dry juniper wood will be 1.3- 1.5 percent, or 26-30 pounds per ton.
2. If operating pressures under 10 psi are used for steam distillation, the oils will naturally deposit cedrol crystals on standing. The yield of. crystalline material is roughly 15 per cent of the oil so cedrol recoveries would be roughly 3.9 to 4.5 pounds per ton of dry wood. At the quoted price of from $3.50 to $4.00 per pound, this would amount to a gross value of from $13.60 to $18.00 per ton of dry wood. This is exclusive of the liquid portions of the oil mainly cedrene.
3. If higher steam pressures are used for distillation, the amount of cedrol recoverable is increased although the crystallization process is more difficult. Using an average yield of 25 percent from the oil, the potential recovery would be from 6.5 to 7.5 pounds per ton of dry wood. The gross return would be from $22.70 to $30.00 per ton of wood. Again, the liquid fraction of the oil remains as a byproduct.
4. If the crude oil is vacuum fractionated, the maximum cedrol yield is obtained, roughly 40 percent of the oil. This would make a gross return of from $36.50 to $48.00 per ton of dry wood for the cedrol alone.
5. If crude oil is the end product of the process, the possible return would be from $13.00 to $15.00 per ton of wood. This is based on current market prices for cedarwood oils which are about 50 cents per lb.
6. The cost of steam is quite low for the processing. Steam costs would probably be about 50 cents per 1000 pounds of steam. Since the estimated consumption is 3000 pounds per ton of wood, the cost would be only $1.50 per ton of wood. The steam cost would be further reduced if spent wood could be used as boiler fuel.
7. No attempt has been made to estimate the cost of procuring juniper wood since the nature of the logging operation would influence the cost considerably.
It is concluded therefore that recovery of crude oil as the end product is probably the least profitable of the possible processes. The recovery of crystalline cedrol offers more promise since the cedrol commands a good price, and the liquid fraction of the oil remains as a byproduct which should have some substantial value.
The use of the spent wood for fiber production would also enhance the economics of the process.
Return to Table of Contents
Literature Cited
Kurth, E.F. and Lackey, H.B. 1948. The constituents of Sierra Juniper Wood (Juniperus occidentalis, Hooker). Journal of the American Chemical Society, 70, 2206-2209.
Burnet, Don. 1954. An Attempt to Recover Cedarwood Oil from Western Juniper by Destructive Distillation. Master of Science Thesis, Oregon State College.
Guenther, E. The Essential Oils, D. Van Nostrand Co., Inc., New York. Vol. I, 1948, p. 472. Vol. II, 1949, p. 852.
Huddle, A.B. 1936. "Oil from Tennessee Red Cedar." Industrial and Engineering Chemistry, 28, 18-21.
Return to Table of Contents
Appendix
Data in this section are included to provide means of calculating theoretical steam requirements for distillation of cedrol. Indications were that 50-60 psi was probably the practical maximum pressure.
Theoretical Calculations for-Steam Distillation of Cedrol and Cedrene:
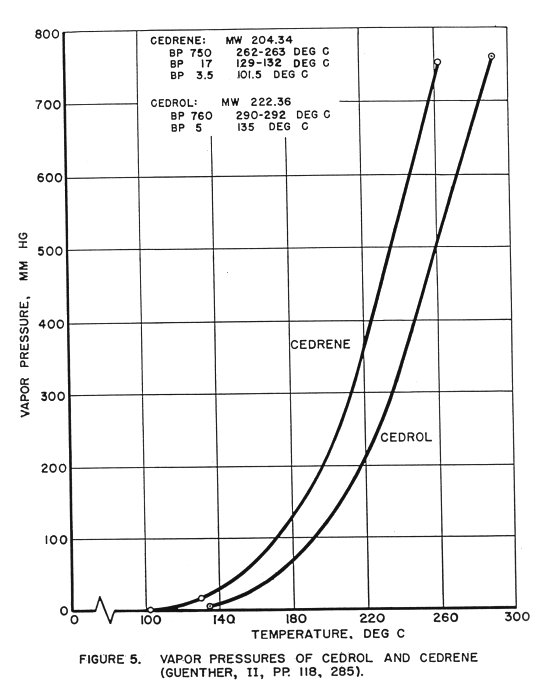
Table 9. Vapor-pressure Curves for Cedrene and Cedrol,
Plotted by Method of Duhring (Figures 5, 6).
| Material | Boiling point | Vapor pressure | Temp. at which water has same vapor pressure |
| Deg C | mm Hg | Deg C | |
| Cedrene | 262 130 102 | 750 17 3.5 | 99.6 19.5 -3.6 |
| Cedrol | 291 135 | 760 5 | 100 1.2 |
(Guenther, pp. 118, 285, Volume II)
Table 10. Calculation of Theoretical Steam Required for Distillation of Cedrol.
| Operating Pressure | Temperature | Vapor pressure of water | Temp from Duhring plot | Vapor pressure of cedrol | ||
| Psig | Lb abs | Deg F | Deg C | mm Hg | Deg C | mm Hg |
| 0 | 14.7 | 212 | 100 | 760 | -27 | 0.6 |
| 10 | 24.7 | 239 | 115 | 1275 | -13 | 1.7 |
| 20 | 34.7 | 259 | 126 | 1790 | -4 | 3.4 |
| 30 | 44.7 | 274 | 135 | 2310 | 1.2 | 5.0 |
| 40 | 54.7 | 286 | 141 | 2830 | 8 | 8.05 |
| 50 | 64.7 | 297 | 148 | 3340 | 12.5 | 10.9 |
| 60 | 74.7 | 307 | 153 | 3860 | 16 | 13.6 |
| 80 | 94.7 | 324 | 162 | 4900 | 21 | 18.7 |
| 100 | 114.7 | 338 | 171 | 5930 | 26.5 | 25.9 |
Calculating theoretical steam required to distill cedrol, using conventional steam distillation equation:
Weight Water = Pw (MWw)
Weight Cedrol Pc (MWc)
MWw = 18 (molecular weight of water)
MWc = 222.4 (molecular weight of cedrol)
Pw = vapor pressure of water
Pc = vapor pressure of cedrol
Partial pressure of cedrol was neglected in the lower range.
At pressures up to 100 psi, steam required is as follows:
| Pressure | Steam required |
| Psi | Lb/ lb cedrol |
| 0 | 102.5 |
| 10 | 60.6 |
| 20 | 42.5 |
| 30 | 37.3 |
| 40 | 28.3 |
| 50 | 24.7 |
| 60 | 22.9 |
| 80 | 21.1 |
| 100 | 18.4 |